シンポジウム「転移性肝癌に挑む」ではProf. Meijerink が大腸癌肝転移に対する外科的切除とアブレーションのランダム化比較試験 の中間解析結果を報告します【第58回日本肝癌研究会】
第58回日本肝癌研究会を『 Lead the World:新たな肝癌診療モデルの構築 』をテーマに、5月12日(木)、13日(金)
http://
シンポジウム4「転移性肝癌に挑む」
「切除可能な大腸肝転移(CRLM)
外科的切除は院内死亡率が高く、有害事象も高頻度だった。
抄録は、印刷締切りまでに届かなかったため、
また、Prof. Meijerinkは、5月12日のランチョンセミナーでもCOLLISION trialについて講演します。
The rapidly expanding role of thermal ablation in the treatment of colorectal liver metastases
Martijn R. Meijerink
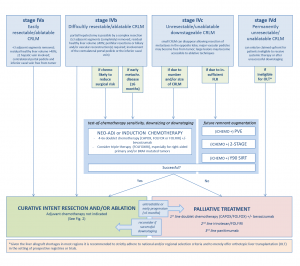
The current standard to treat resectable colorectal liver metastases (CRLM) is surgical resection. Guidelines currently reserve thermal ablation for anatomically unresectable metastases and for patients whose comorbidities disqualify them as surgical candidates. However, looking at the preplanned interim results of the ongoing phase III randomized controlled COLLISION trial (ClinicalTrials.gov, NCT03088150), that paradigm is very likely to change in the near future.
For unresectable disease, the highest achievable level of evidence seems to have been reached with the publication of the long-term results of the EORTC-CLOCC trial.1 Thermal ablation (± surgical resection) plus chemotherapy versus chemotherapy alone showed a remarkable difference in 8-year overall survival of 35.9% versus 8.9%, respectively. The results irrefutably demonstrated that aggressive local therapy can prolong survival or in a subset of patients even provide cure. As a consequence, further randomized comparisons of local ablative therapy to palliative intent chemotherapy alone should be considered unethical.2
Two recently published systematic reviews and one meta-analysis enumerated all available comparative retrospective series regarding thermal ablation and partial hepatectomy in the treatment of CRLM.2,3 In both reviews, thermal ablation demonstrated significantly fewer complications, but also an inferior overall survival. However, both author groups concluded that the high risk of residual confounding, when comparing surgical candidates to disease not amenable for complete surgical eradication, renders it impossible to make hard conclusions and both state that a randomised controlled non-inferiority trial is urgently needed to answer the time-honored question whether we should resect or ablate small-size CRLM. The European, but predominantly Dutch COLLISION trial group, embedded within the Dutch Colorectal Cancer Group and funded by an unrestricted grant from Medtronic – Covidien, managed to accrue, randomize and treat 200 patients within 3.5 years and the as of yet unpublished results of the preplanned interim analysis with futility were recently presented at the ECIO 2022 in Vienna.4
Surgical resection was associated with a higher in-hospital mortality and a higher number of low- and high-grade adverse events (p = 0.010). Length of hospital stay was shorter for thermal ablation (median stay 5 days for resection and 2 days for ablation; p = < 0.001). No differences were found with regard to local and distant tumor progression-free survival and local control was superior following thermal ablation. With a conditional probability of 88.3% to prove non-inferiority for the primary endpoint overall survival, the futility threshold was amply exceeded. The group will continue recruitment, pending a new interim analysis with boundaries to stop for efficacy planned one year after having randomized half of the initial sample-size (n = 309). The final results are expected in approximately 3 years.
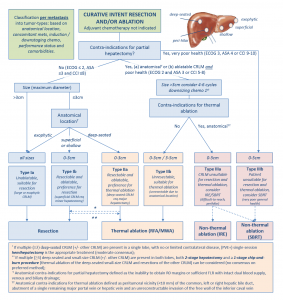
In the meantime the recently published multidisciplinary resectability and ablatability criteria from the COLLISION trial group can help tumor board decisions whether to opt for surgery, thermal or non-thermal ablation.5
- Ruers T, Van Coevorden F, Punt CJ, et al: Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 109, 2017
- Meijerink MR, Puijk RS, van Tilborg A, et al: Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol 41:1189-1204, 2018
- van Amerongen MJ, Jenniskens SFM, van den Boezem PB, et al: Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases – a meta-analysis. HPB (Oxford) 19:749-756, 2017
- Puijk RS, Ruarus AH, Vroomen L, et al: Colorectal liver metastases: surgery versus thermal ablation (COLLISION) – a phase III single-blind prospective randomized controlled trial. BMC Cancer 18:821, 2018
- Nieuwenhuizen S, Puijk RS, van den Bemd B, Aldrighetti L, Arntz M, van den Boezem PB, et al. Resectability and Ablatability Criteria for the Treatment of Liver Only Colorectal Metastases: Multidisciplinary Consensus Document from the COLLISION Trial Group. Cancers (Basel) 2020;12(7)
(Figures はクリックすると拡大します)